MD09
CASE OF THE MONTH – APRIL 2022
Case Presentation:
EMS is called to the home of a 38-year-old male with a past medical history of heart failure complaining of severe, sudden onset headache which started several hours prior. Per his family, he has had no recent trauma, illness, vomiting, syncope or focal weakness that they have observed, however they do say that he has seemed more confused as the day has progressed. Paramedics proceed to find an adult male sitting on the couch, in moderate distress complaining of headache, nausea and vomiting. He ambulates to the stretcher for you with intact, but slow gait, holding his left ventricular assist device (LVAD).
A Ventricular Assist Device (VAD) is a Mechanical Circulatory Support (MCS) device used primarily as a bridge to heart transplant when medical therapy is no longer able to control end stage heart failure. The LVAD, for patients with primarily left heart failure, works by connecting an inflow cannula from the left ventricle to a pump, which then flows into an outflow graft connected to the aorta. The device essentially works as an artificial “pump” by replacing the function of the failed ventricle.

Figure 1. Left ventricular assist device. An inflow cannula connects the left ventricle to a battery-operated pump that flows into an outflow cannula that flows into the heart. Creative Commons Image (CC BY-SA 3.0): https://en.wikipedia.org/wiki/Ventricular_assist_device#/media/File:Blausen_0621_LVAD.png
MCG 1325, Mechanical Circulatory Support Devices also discusses right ventricular assist devices (RVADs), biventricular devices, and total artificial hearts (TAHs) which are less common, but good to be familiar with. The most commonly encountered device is the LVAD. These devices may be seen with increased frequency as many studies have shown it may be a reliable option as a bridge to definitive recovery or heart transplant for many patients or may be permanent for patients that are not candidates for transplant and are unlikely to recover cardiac function.
There are several different MCS device manufacturers and devices available today. In general, the principles are the same. Outside of the body in a patient with a MCS device you will see the driveline – an encased, bundle of wires exiting from the upper abdomen, which connects to the controller box. The controller box monitors pump function, records pump data, drives power supply, and has a display screen for alarms and battery life. All monitor and pump function data are stored within the machine and tracked by the device program. Patients will typically have a stabilization belt to support and anchor the drive line and controller box with two batteries always attached. The batteries can be recharged with a standard power supply.
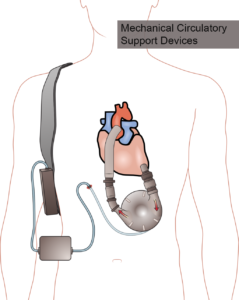
Figure 2. Left ventricular assist device. An inflow cannula connects the left ventricle to a battery-operated pump that flows into an outflow cannula that flows into the heart. The driveline, controller box, and battery are external. Creative Commons Image (CC BY-SA 3.0):
https://en.wikipedia.org/wiki/Ventricular_assist_device#/media/File:Ventricular_assist_device.png
After taking a careful history, locate a tag on the controller box indicating the type of device, the hospital where it was placed, and the contact number for the MCS coordinator, available 24 hours daily. Always check battery life and for any alarms prior to transporting. If there is any concern for device malfunction, contact the MCS coordinator immediately. If the coordinator is unable to be reached, make Base Contact for direction. When transporting MCS patients, take every precaution to ensure their device and driveline are secured to them and all equipment accompanies them. Attempt to locate an Advanced Healthcare Directive and/or Standardized Patient-Designated Directives (e.g. POLST, state DNR form) as many MCS patients have made end-of-life care decisions.
MCS device patients must be assessed as would any other patient. The underlying condition that prompted the EMS call may be related or unrelated to the device. Most MCS device patient emergencies will NOT be related to malfunction of the device. However, the presence of the MCS device may require EMS to make adjustments to the patient assessment.
Most VAD’s are continuous flow meaning there will be no palpable pulse. However auscultating over the epigastrium should reveal a subtle mechanical flow sound to confirm device is active. The lack of pulsation makes standard blood pressure measurements unreliable, and the patient should first be assessed hemodynamically with objective measures such as extremity perfusion and mental status. Due to the continuous flow, in addition to absent pulse, the patient’s heart rate and pulse oximetry may be unobtainable or unreliable. Capnography can be used as an EtCO2 <20mmHg reliably indicates poor perfusion. A VAD patient’s “blood pressure” is based on a number similar to mean arterial pressure (MAP) and ideally should be somewhere in the 70-90 mmHg range. To obtain this reading, apply a manual blood pressure cuff in standard fashion and then inflate. During deflation, place a stethoscope over the brachial or radial artery and listen for the return of loud continuous flow sounds. This signifies the MAP. The heart rhythm displayed on a cardiac monitor will be a reliable representation of the patient’s current inherent electrical rate and rhythm. Recall, however, that blood flow is entirely dependent on the VAD itself. This month’s Video of the Month, Mechanical Circulatory Support Devices, discusses these considerations in more detail.
When treating a MCS device patient, the first step is to determine if the patient’s presentation is related to device malfunction. If that is the case, the MCS device coordinator should be contacted immediately. A device malfunction should be suspected if the device is alarming or if the patient appears to be poorly perfused. Once determining the device is working appropriately, assess the patient and determine a provider impression as one would for any other patient.
MCS device patients are usually anticoagulated with warfarin (Coumadin) and antiplatelet agents, putting them at risk for bleeding complications. Any information about their method of anticoagulation including dosing, compliance, and recent lab checks may increase the suspicion for a bleeding complication contributing to the patient’s presentation.
EMS clinicians complete their assessment and are preparing to transport. The patient is alert and oriented x 3, GCS 14, MAP 82mmHg in the right arm, RR 18. A pulse and pulse oximetry cannot be determined. He is afebrile. The patient is uncomfortable, reporting 8/10 pain and nauseated. His skin is well perfused, warm and dry. His pupils are equal, round, and reactive, mLAPSS negative, LAMS 0, LKWT 12 hours ago. Glucose is 88. The patient can ambulate to the stretcher and displays repetitive questioning and confusion which his family insist is not his baseline.
Although the patient is alert and oriented, he is displaying an obvious deviation from his baseline mental status which is corroborated by his family. The primary provider impression should be ALOC – Not Hypoglycemia or Seizure (ALOC) with a secondary provider impression of Headache – Non-Traumatic (HPNT). The patient should be managed in accordance with TP-1229, Altered Level of Consciousness and TP-1202, General Medical. The provider impression ALOC requires Base Contact when there is a persistent unclear etiology to discuss potential underlying causes that would impact transport destination.
MCS device patients may present with ALOC if they are poorly perfused due to a mechanical issue with the MCS device, however in this patient, the initial assessment reveals the device to be functioning well as there are no alarms, continuous flow sounds with epigastric auscultation, and good perfusion with a normal MAP. The cause for ALOC is likely unrelated to the mechanical function of the LVAD. His pain and nausea can be treated in accordance with TP-1202, General Medical. After confirming a negative stroke assessment (mLAPSS), no trauma, and no underlying causes that can be treated by EMS for his ALOC (e.g. hypoglycemia, poor perfusion, etc.), transport the patient to the MAR.
The patient is transported to the MAR where his presentation raises concern for intracranial hemorrhage (ICH). A CT scan is performed demonstrating a spontaneous intraparenchymal hemorrhage (bleeding within the brain tissue). The increased intracranial pressure from the hemorrhage caused the headache, nausea and subsequent ALOC. The patient required surgery and given the complexities of anesthesia with the MCS device, transport to a tertiary care center with MCS device specialists and neurosurgical capabilities was indicated. The patient required intubation in the emergency department for airway protection due to declining mental status and was transferred via critical care transport to his MCS device hospital for specialty care.
- When managing an MCS device patient, the first step is to determine if their device has malfunctioned. Most MCS device patient’s emergencies will NOT be related to malfunction of the device, but if a device malfunction is suspected, contact the MCS device coordinator. For device malfunction, the patient must be transported to the coordinating center.
- LVAD patients have continuous, non-pulsatile flow making vitals signs such as blood pressure, heart rate, and pulse oximetry unobtainable or unreliable. Assessments should be based on the patient’s perfusion status and clinical exam. Capnography will be accurate and can provide information on perfusion status.
- MCS device patients are usually anticoagulated and are at risk for bleeding complications.
- Sudden onset headaches may be a presentation for spontaneous intracranial hemorrhage. Nausea and vomiting may be an accompanying symptom for this emergent condition.
Author: Ryan DeVivo, DO